

 |
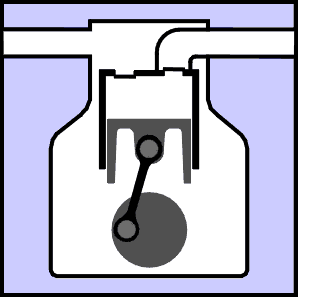
This section explains in basic terms the principals that are used to create the refrigeration effect. Graphics and animation's are used in an attempt to make it easy to understand the concepts involved. First of all, did you know that there is no such thing as cold? You can describe something as cold and everyone will know what you mean, but cold really only means that something contains less heat than something else. All there really is, is greater and lesser amounts of heat. The definition of refrigeration is The Removal and Relocation of Heat. So if something is to be refrigerated, it is to have heat removed from it. If you have a warm can of pop at say 80 °F and you would prefer to drink it at 40 °F you could place it in your fridge for a while, heat would somehow be removed from it, and you could eventually enjoy a less warm pop. (oh, all right, a cold pop.) But lets say you placed that 40 °F pop in the freezer for a while and when you removed it, it was at 35 °F See what I mean, even "cold" objects have heat content that can be reduced to a state of "less heat content". The limit to this process would be to remove all heat from an object. This would occur if an object was cooled to Absolute Zero which is -460 ° F or -273 ° C. They come close to creating this temperature under laboratory conditions and strange things like electrical superconductivity occur. |
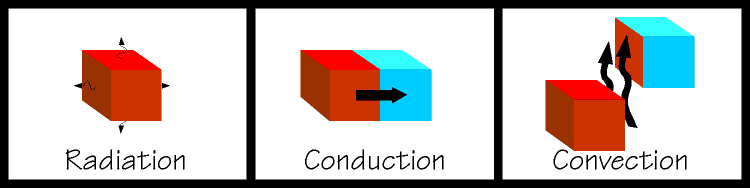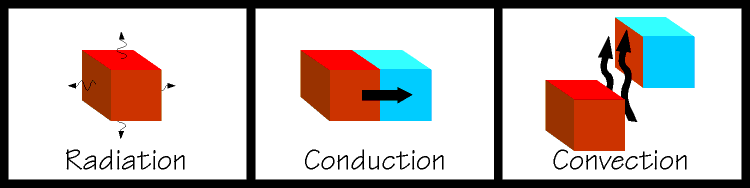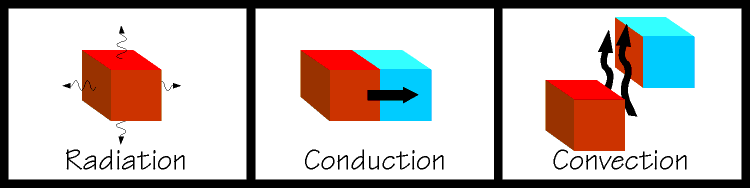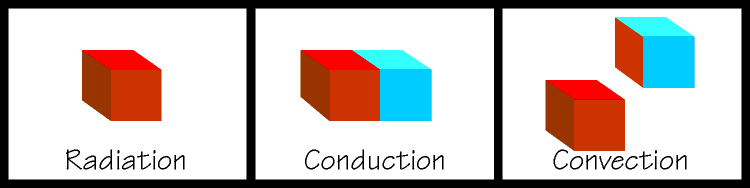
| The latter two are used extensively in the design of refrigeration equipment. If you place two objects together so that they remain touching, and one is hot and one is cold, heat will flow from the hot object into the cold object. This is called conduction. This is an easy concept to grasp and is rather like gravitational potential, where a ball will try to roll down an inclined plane. If you were to fan a hot plate of food it would cool somewhat. Some of the heat from the food would be carried away by the air molecules. When heat is transferred by a substance in the gaseous state the process is called convection. And if you kicked a glowing hot ember away from a bonfire, and you watched it glowing dimmer and dimmer, it is cooling itself by radiating heat away. Note that an object does not have to be glowing in order to radiate heat, all things use combinations of these methods to come to equilibrium with their surroundings. So you can see that in order to refrigerate something, we must find a way to expose our object to something that is colder than itself and nature will take over from there. We are getting closer to talking about the actual mechanics of a refrigerating system, but there are some other important concepts to discuss first. |
The States of MatterThey are of course; solid, liquid and gas. It is important to note that heat must be added to a substance to make it change state from solid to liquid and from liquid to a gas. It is just as important to note that heat must be removed from a substance to make it change state from a gas to a liquid and from a liquid to a solid. |
The Magic of Latent Heat |
||
| BTU | ||
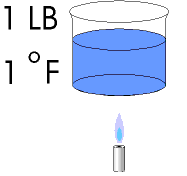 |
Long ago it was found that we needed a way to quantify heat. Something more precise than "less heat" or "more heat" or "a great deal of heat" was required. This was a fairly easy task to accomplish. They took 1 Lb. of water and heated it 1 degree Fahrenheit. The amount of heat that was required to do this was called 1 BTU. (British Thermal Unit). The refrigeration industry has long since utilized this definition. You can for example purchase a 6000 BTUH window air conditioner. This would be a unit that is capable of relocating 6000 BTU's of heat per hour. A unit with a capacity of 12,000 BTUH would be called a one Ton unit. There are 12,000 BTU's in 1 Ton. | |
| To raise the temperature of 1 lb
of water from 40 °F to 41 °F would take 1 BTU.
To raise the temperature of 1 lb of water from 177 °F to 178 °F would also take 1 BTU.
However, if you tried raising the temperature of water from 212 °F to 213 °F you would not be able to do it. Water boils at 212 °F
and would prefer to change into a gas rather than let you get it any hotter. Something of utmost importance occurs at the boiling
point of a substance. If you did a little experiment and added 1 BTU of heat at a time to 1 lb
of water, you would notice that the water temperature would increase by 1 degree Fahrenheit each time. That is until you reached 212 °F
Then something changes. You would keep adding BTU's, but the water would not get any hotter! It would change state into a gas and
it would take 970 BTU's to vapourize that entire pound of water. This is called the Latent Heat of Vapourization and in
the case of water it is 970 BTU's per pound.
So what you say! When are you going to tell me how the refrigeration effect works? Well hang in there, you have just learned about 3/4 of what you need to know to understand the process. What keeps that beaker of water from boiling when it is at room temperature? If you say it's because it is not hot enough, sorry but you are wrong. The only thing that keeps it from boiling is the pressure of the air molecules pressing down on the surface of the water. When you heat that water to 212 °F and then continue to add heat, what you are doing is supplying sufficient energy to the water molecules to overcome the pressure of the air pressing down on it's surface and allow them to escape from the liquid state. If you took that beaker of water to outer space where there is no air pressure the water would flash into a vapour instantly. If you took that beaker of water to the top of Mt. Everest where there is much less air pressure than at lower altitudes, you would find that much less heat would be needed to boil the water. (it would boil at a lower temperature than 212 °F So water boils at 212 °F at normal atmospheric pressure. Lower the pressure and you lower the boiling point. Therefore we should be able to place that beaker of water under a bell jar and have a vacuum pump extract the air from within the bell jar and watch the water come to a boil even at room temperature. This is indeed the case! A liquid requires heat to be added to it in order for it to overcome the air pressure pressing down on its' surface if it is to evaporate into a gas. We just learned that if the pressure above the liquids surface is reduced it will evaporate easier. We could look at it from a slightly different angle and say that when a liquid evaporates it absorbs heat from the surrounding area. So, finding some fluid that evaporates at a handier boiling point than water (i.e. lower) was one of the first steps required for the development of mechanical refrigeration. Chemical Engineers spent years experimenting before they came up with appropriate chemicals for the job. They developed a family of hydroflourocarbon refrigerants which had extremely low boiling points. These chemicals would boil at temperatures below 0 °F at atmospheric pressure. So finally, we can begin to describe the mechanical refrigeration process. |
||